The Paccific-- - IP
New Gene Therapy Lowers Eye Pressure To Treat Glaucoma
The high eye pressure seen in glaucoma slowly leads to blindness. For some, the first-line treatment, eye drops, doesn't work. Researchers have used gene therapy to develop a promising new way of treating the high eye pressure associated with glaucoma.
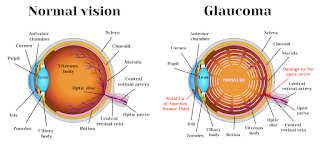
Affecting up to 80 million people worldwide, glaucoma is usually caused by raised intraocular pressure (IOP). The number of people with glaucoma is expected to rise to 110 million by 2040.
The eye constantly produces a liquid called aqueous humor, which helps the eye hold its shape and nourishes the eye. The fluid is drained out of the eye through the anterior chamber angle or drainage angle. If the drainage angle is damaged, the eye produces more aqueous humor than it can drain, causing high IOP that can irreversibly damage the optic nerve, leading to blindness.
The first-line treatment for glaucoma is eye drops made of a prostaglandin analog, which lowers IOP. However, 25% to 50% of people don't respond to the treatment, and their eye pressure remains elevated.
Researchers at Trinity College Dublin have collaborated with the biotechnology company Exhaura Ltd to develop a novel gene-therapy-based approach to decreasing IOP that shows great promise in the treatment of glaucoma.
"This exciting project allowed us to bridge the gap between academia and industry and work very closely with a gene therapy company to develop a cutting-edge therapy that we believe holds immense promise for patients in the future," said Matthew Campbell, corresponding author of the study.
The researchers used an adeno-associated virus (AAV), a bioengineered tool that uses a non-enveloped virus to deliver modified genetic material into tissues and cells. After delivery, the modified genes create new instructions for those tissues or cells, helping to treat disease.
Here, researchers used AAV to deliver instructions to produce the enzyme matrix metalloproteinase-3 (MMP-3), which helps to kickstart the outflow of aqueous humor from the eye.
The researchers started their experiments on mice, injecting AAV into the back of the eye. They found that the increase in MMP-3 mediated by the AAV increased the outflow of the fluid and decreased IOP. When they tested the therapy on human donor eyeballs, they also found that outflow increased.
The researchers say that the years of research undertaken to get to this point were worth it and that the study's findings are promising.
"Our novel approach to treating glaucoma using gene therapy is the culmination of over seven years of research," said Jeffrey O'Callaghan, lead author of the study. "We are hopeful that this therapy will pave the way to the development of treatments for other forms of blinding eye diseases."
The study was published in the journal Science Advances.
Source: Trinity College Dublin
Gene Therapy Could Help Treat Millions Affected With Glaucoma

Pictoral differences between normal eye and one affected by glaucoma
Currently, topical eye drops are used to prevent the disease from progressing in glaucoma patients. However, there is also a subset of patients who develop resistance to the treatment.
Researchers at the Smurfit Institute of Genetics at Trinity College used a virus to infect the eye cells. The virus was genetically modified to include a gene for the synthesis of an enzyme called metalloproteinase-3, or MMP-3. The enzyme is a matrix protein and results in an increase in the flow of aqueous fluid from the front of the eye, thereby reducing the pressure inside.
The researchers conducted experiments in multiple models of the disease as well as in donor human eyes to test the efficacy of the treatment. Since the research was carried out in partnership with the industry, the experimental outputs were all directed toward translating the results into a clinical program for regulatory purposes.
"This exciting project allowed us to bridge the gap between academia and industry and work very closely with a gene therapy company to develop a cutting-edge therapy that we believe holds immense promise for patients in the future," said Professor Matthew Campbell, Professor in Genetics at Trinity.
Glaucoma Drugs Prevent Cognitive Impairment Associated With Alzheimer's Disease In Mice
Summary: Drugs used to treat glaucoma and high-altitude sickness may prevent cognitive impairment caused by Alzheimer's disease in mice. The research indicates that carbonic anhydrase inhibitors, already approved by the US Food and Drug Administration (FDA), promote the clearance of amyloid beta from blood vessels and glial cells, which control brain inflammatory processes, as well as reducing inflammation, restoring cell function and preventing cognitive impairment.
Key Points:
Source: Temple University Health System
Changes in blood vessels in the brain linked to the build-up of a sticky protein known as amyloid beta are a hallmark of early-stage Alzheimer's disease.
As amyloid accumulates on the walls of vessels, brain cells lose nutrients and oxygen, becoming inflamed and dysfunctional. Over time, this gives rise to cerebral amyloid angiopathy (CAA), a major cause of aging-related cognitive decline.
Reversing the effects of CAA and neuroinflammation could bring significant benefits for individuals at risk of Alzheimer's disease – and now, new research at the Lewis Katz School of Medicine at Temple University brings that hope within reach.
In experiments carried out in mice, the Temple scientists show that drugs known as carbonic anhydrase inhibitors (CAIs), already FDA-approved for other conditions, such as glaucoma and high-altitude sickness, promote the clearance of amyloid beta from blood vessels and glial cells, which control brain inflammatory processes.
In doing so, CAIs not only reduce inflammation and restore cell function but also prevent cognitive impairment.
The study took place in the lab of Silvia Fossati, PhD, Associate Professor of Neural Sciences and Cardiovascular Sciences at the Lewis Katz School of Medicine at Temple University, and is the first to test the FDA-approved CAIs acetazolamide and methazolamide in animals with cerebrovascular alterations mimicking those of CAA and Alzheimer's disease in humans.
The results appeared online in the journal Alzheimer's & Dementia: The Journal of the Alzheimer's Association.
"Cognitive impairment is closely associated with damage to blood vessels and inflammation in the brain, which are among the main causes, but also consequences, of amyloid accumulation," explained Dr. Fossati, who is also Associate Director of the Alzheimer's Center at Temple's Katz School of Medicine.
"We wanted to see if we could prevent cerebrovascular dysfunction, inflammation and improve cognitive function by therapeutically improving the health of vascular and glial cells, thereby facilitating the removal of amyloid from blood vessels and the brain tissue."
In previous work, Dr. Fossati and colleagues found that amyloid accumulation causes mitochondria – the energy-generating powerhouses of cells – to function abnormally and that this process is linked to activity of the enzyme carbonic anhydrase.
"We also know from experiments in cells that CAIs suppress mitochondrial dysfunction and cell death induced by amyloid," Dr. Fossati said. "But whether these same effects occur in vivo has been unknown."
To test CAIs in vivo, Dr. Fossati and colleagues used a mouse model in which animals, as they age, exhibit increasing levels of human amyloid protein in the brain. As amyloid deposits accumulate in the brain vasculature, the animals begin to show signs of cerebrovascular dysfunction, similar to the way in which humans develop signs of CAA.
The animals were treated with either acetazolamide or methazolamide from about 8 months of age, when signs of amyloid pathology first emerge in these animals, until about 15 or 16 months, when advanced cognitive impairment is present.
When the researchers examined brain tissue from CAI-treated mice, they found significant reductions in amyloid in the cerebral vasculature and in glial cells. They also found that glial cells and blood vessels were overall healthier and had better amyloid-clearing capacity compared to untreated animals.
"Both acetazolamide and methazolamide were highly effective in reducing amyloid deposition and in improving cerebrovascular function," Dr. Fossati said.
"Our behavioral studies showed that, as Alzheimer's pathology decreased, CAI-treated mice experienced noticeable gains in cognitive function."
Additional analyses focused on post-mortem human brain tissue from Alzheimer's patients. These analyses showed that, similar to animals, levels of a specific carbonic anhydrase enzyme found in mitochondria are abnormally elevated in the brains of Alzheimer's disease and CAA patients. The evidence is the first to identify carbonic anhydrase as a key factor in humans affected by these conditions.
The group plans next to design and investigate CAIs that are more specific to mitochondria, with the help of Marc Ilies, PhD, Professor in the Department of Pharmaceutical Sciences at the Temple School of Pharmacy.
"The therapies we used for the current study target carbonic anhydrases in the whole cell," Dr. Fossati explained. "If we can target the enzyme specifically within mitochondria, the efficacy of therapy could improve greatly, and side effects could be reduced."
Nonetheless, clinical trials to test the effectiveness of acetazolamide and methazolamide against CAA in humans could be underway soon, as both agents are already approved by the FDA.
Other researchers who contributed to the new study include Elisa Canepa, Rebecca Parodi-Rullan, Rafael Vazquez-Torres, Roberto Guzman-Hernandez, Nicole L. Lemon, and Federica Angiulli, Alzheimer's Center at Temple, Department of Neural Sciences, Lewis Katz School of Medicine; Begona Gamallo-Lana and Adam C. Mar, Department of Neuroscience and Physiology, Neuroscience Institute, NYU Grossman School of Medicine; Ludovic Debure and Thomas Wisniewski, Department of Neurology, Center for Cognitive Neurology, NYU Grossman School of Medicine; Marc A. Ilies, Department of Pharmaceutical Sciences and Moulder Center for Drug Discovery Research, Temple University School of Pharmacy; and Leif Østergaard and Eugenio Gutiérrez-Jiménez, Center of Functionally Integrative Neuroscience (CFIN), Department of Clinical Medicine, Aarhus University, Denmark.
Funding: Funding was provided in part by grants from the National Institutes of Health, the Edward N. And Della L. Thome Memorial Foundation Awards Program in Alzheimer's Disease Drug Discovery Research, the Alzheimer's Association, the Pennsylvania Department of Health Collaborative Research on Alzheimer's Disease (PA Cure), the Karen Toffler Charitable Trust, and the Lemole Center at Temple.
Author: Jeremy WalterSource: Temple University Health SystemContact: Jeremy Walter – Temple University Health SystemImage: The image is credited to Neuroscience News
Original Research: Open access."FDA-approved carbonic anhydrase inhibitors reduce amyloid β pathology and improve cognition, by ameliorating cerebrovascular health and glial fitness" by Silvia Fossati et al. Alzheimer's & Dementia
Abstract
FDA-approved carbonic anhydrase inhibitors reduce amyloid β pathology and improve cognition, by ameliorating cerebrovascular health and glial fitness
IntroductionCerebrovascular pathology is an early and causal hallmark of Alzheimer's disease (AD), in need of effective therapies.
MethodsBased on the success of our previous in vitro studies, we tested for the first time in a model of AD and cerebral amyloid angiopathy (CAA), the carbonic anhydrase inhibitors (CAIs) methazolamide and acetazolamide, Food and Drug Administration–approved against glaucoma and high-altitude sickness.
ResultsBoth CAIs reduced cerebral, vascular, and glial amyloid beta (Aβ) accumulation and caspase activation, diminished gliosis, and ameliorated cognition in TgSwDI mice. The CAIs also improved microvascular fitness and induced protective glial pro-clearance pathways, resulting in the reduction of Aβ deposition. Notably, we unveiled that the mitochondrial carbonic anhydrase-VB (CA-VB) is upregulated in TgSwDI brains, CAA and AD+CAA human subjects, and in endothelial cells upon Aβ treatment. Strikingly, CA-VB silencing specifically reduces Aβ-mediated endothelial apoptosis.
DiscussionThis work substantiates the potential application of CAIs in clinical trials for AD and CAA.

Comments
Post a Comment