Sarcoidosis: Causes, Symptoms, Diagnosis & Treatment
Increased Risk Of POAG Associated With Polygenic Risk Score
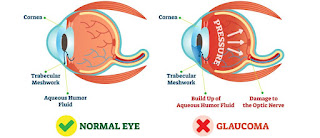
Polygenic risk score was found to be associated with a higher risk of primary open angle glaucoma (POAG) in patients with ocular hypertension.
Patients with ocular hypertension had an increased risk of primary open angle glaucoma (POAG) if they had a higher polygenic risk score (PRS), according to a study published in JAMA Ophthalmology. Prediction of POAG was also found to improve with the inclusion of PRS.
POAG accounts for about 69% of incidences of glaucoma, one of the leading causes of blindness. Intraocular pressure (IOP) has been the only risk factor for the development and progression of POAG that can be altered. POAG is also a heritable disease, which can be evaluated with the PRS using an aggregate of overall genetic burden. This study aimed to use data from the Ocular Hypertension Treatment Study (OHTS) to create a PRS and if POAG risk was linked to underlying genetic risk outside of demographic and ocular factors.
Close-up of eyesImage credit: Alessandro Grandini - stock.Adobe.Com
OHTS was a randomized clinical trial that assessed the efficacy of topical therapy that lowered IOP. There were 1636 participants with ocular hypertension (OHTN) from 22 sites in the United States who were included in the study and randomization began in 1994 and ended in June 2002 for phase 1; phase 2 began in June 2002 and last until March 2009 and phase 3 began in July 2015 and ended in June 2020. A total of 1077 participants also participated in the ancillary genetics study. Most of the participants had European or African ancestry.
The POAG PRS was created using summary statistics from a cross-ancestry POAG genome-wide association study meta-analysis. A total of 8,813,496 genomic variants from 449,186 cross-ancestry participants in the UK Biobank were used to train the PRS. A baseline regression analysis was used for predicting the time from conversion of OHTN to POAG.
Overall, there were 1009 participants included for this study, of which 55.7% were female and the mean (SD) age was 55.9 (9.3) years. There were 287 eyes in 212 participants who developed POAG.
Participants who contracted POAG has a significantly higher mean (SD) PRS score (0.24 [0.95]) compared with participants who didn't develop POAG (–0.12 [1.00]). Mean PRS was higher in participants of European ancestry who contracted POAG (0.29 [0.95]) compared with the participants of European ancestry who did not contract POAG (–0.13 [0.99]). This trend was not found in those of African ancestry, as those who contracted POAG did not have a significantly higher PRS compared with those who did not contract POAG (0.13 [0.93] vs –0.10 [1.04]).
Those in the lowest and highest baseline risk tertiles did not have a significant difference in mean PRS overall as well as those specifically of European and African ancestry. However, a significantly increased risk of POAG onset was found in the highest tertile of PRS compared with those in the lowest at the end of phase 1 (3.01-fold, 5.93% absolute increase), phase 2 (2.30-fold, 9.96% absolute increase), and phase 3 (2.00-fold, 9.93% absolute increase). This extended to those of European and African ancestry.
A decile higher PRS score was associated with a greater risk of POAG conversion per eye equivalent to 2.46% (95% CI, 2.10-2.81). A linear regression found an increase from 14.85% (95% CI, 11.73%-17.98%) conversion in 20 years in the lowest decile to 36.99% (95% CI, 33.86%-40.11%) conversion in the highest. Each decile was associated with a greater conversion risk for POAG equivalent to 1.36% (95% CI, 1.08%-1.64%).
There were some limitations to this study. POAG could have been classified incorrectly in the UK Biobank, which could result in poor training of the PRS. The sample size was relatively small and had a low proportion of participants who had POAG. Participants were also lost to follow-up over the course of 15 years. Generalizability of the study would be improved from validation of the PRS in external study populations.
The researchers concluded that a high PRS score for POAG was associated with an increased risk for the eye condition in patients with OHTN.
Reference
Singh RK, Zhao Y, Elze T, et al. Polygenic risk scores for glaucoma onset in the ocular hypertension treatment study. JAMA Ophthalmol. Published online March 14, 2024. Doi:10.1001/jamaophthalmol.2024.0151
Higher Polygenic Risk Score Tied To Increased Risk For Glaucoma
Higher polygenic risk score (PRS) was associated with an increased risk for primary open-angle glaucoma (POAG) in patients with ocular hypertension, according to a post-hoc analysis of the Ocular Hypertension Treatment Study.
Among over 1,000 patients, risk of POAG increased 1.36% with each higher PRS decile, with conversion ranging from 9.52% in the lowest decile to 21.81% in the highest decile, reported Nazlee Zebardast, MD, of Massachusetts Eye and Ear and Harvard Medical School in Boston, and colleagues.
Comparing the low-risk and high-risk PRS tertiles showed a twofold increase in 20-year POAG risk for patients of European and African ancestries, they detailed in JAMA Ophthalmology.
"These findings suggest background genetic risk for POAG may help stratify patients with ocular hypertension and help clinicians and patients make decisions about early treatment," the authors wrote.
Prediction models significantly improved with the addition of PRS as a covariate compared with the study's baseline model (P<0.001), Zebardast and team reported. Each 1-standard-deviation-higher PRS conferred a mean hazard ratio of 1.25 (95% CI 1.13-1.44) for POAG onset.
"POAG is a highly heritable disease, with 127 identified common risk variants to date," they explained. "While each variant individually has a small effect, they can be used in the aggregate to measure overall genetic burden with a PRS. Genotyping can be done inexpensively once per lifetime and enable the calculation of a PRS for a wide range of diseases."
In an accompanying commentary, Fei Li, MD, PhD, and Xiulan Zhang, MD, PhD, of Guangdong Provincial Clinical Research Center for Ocular Diseases in China, noted that "the study's key finding -- that a PRS can independently predict the onset of POAG in individuals with ocular hypertension -- is both groundbreaking and timely."
Current risk analysis strategies often fail to pick up disease until it's advanced to cause irreversible damage, they wrote. "The introduction of PRS as a predictive tool may lead to a paradigm shift, offering the possibility of identifying at-risk individuals before the clinical onset of the disease."
"For instance, patients with a high PRS may benefit from more frequent monitoring, earlier intervention, and possibly more aggressive treatment approaches to prevent or delay the onset of POAG," they added. "Conversely, those with a lower PRS may require less intensive surveillance, potentially reducing healthcare costs and the burden of unnecessary interventions."
Primary findings from the Ocular Hypertension Treatment Study showed that topical ocular hypotensive medication was effective in delaying or preventing the onset of POAG in patients with elevated intraocular pressure.
For this post-hoc analysis of the study, data were taken from 22 U.S. Sites with a mean follow-up of 14 years. A total of 1,636 participants were followed from February 1994 to December 2008 -- 1,077 were enrolled in an ancillary genetics study, and 1,009 met criteria for this analysis.
Of the 1,009 participants, mean age was 55.9, 55.7% were women, 757 were of European ancestry, and 252 were of African ancestry. Mean PRS was significantly higher for the 350 POAG converters compared with the 659 non-converters (0.24 vs -0.12, P<0.001).
PRS was calculated using summary statistics from a cross-ancestry POAG genome-wide association study meta-analysis. The PRS was trained using 8,813,496 variants from 449,186 cross-ancestry participants in the U.K. Biobank.
Zebardast and team acknowledged that the relatively small size of the study cohort, the low proportion of participants with confirmed POAG, and loss to regular follow-up after 15 years were all limitations to the study.
Randy Dotinga is a freelance medical and science journalist based in San Diego.
Disclosures
The study was funded by grants from the National Eye Institute, a Research to Prevent Blindness Career Development Award, an American Glaucoma Society Clinician Scientist Award, and the Glaucoma Foundation.
Zebardast reported grants from the National Eye Institute and Research to Prevent Blindness.
Co-authors reported relationships with the National Institutes of Health, Genentech, the Glaucoma Foundation, the National Eye Institute, Research to Prevent Blindness, Twenty Twenty, Character Bio, and CRISPR Therapeutics and Editas.
Primary Source
JAMA Ophthalmology
Source Reference: Singh RK, et al "Polygenic risk scores for glaucoma onset in the Ocular Hypertension Treatment Study" JAMA Ophthalmol 2024; DOI: 10.1001/jamaophthalmol.2024.0151.
Secondary Source
JAMA Ophthalmology
Source Reference: Li F, Zhang X "The role of polygenic risk scores in glaucoma management" JAMA Ophthalmol 2024; DOI: 10.1001/jamaophthalmol.2024.0220.
Please enable JavaScript to view the comments
ViaLase Wins $40M To Advance Femtosecond Laser For Glaucoma
03 Apr 2024
Californian company founded by ophthalmology laser pioneer Tibor Juhasz backed in series C funding round.
ViaLase, a medical technology startup developing a new femtosecond laser treatment for glaucoma, has closed its series C funding round with $40 million in additional finance.
Based in Alisa Viejo, between Los Angeles and San Diego, ViaLase says that the cash injection will ensure the continued advancement of clinical, regulatory, and commercial milestones of the ultrafast laser approach, which it describes as non-invasive.
"If approved, the ViaLase Laser will be the first femtosecond laser used for the treatment of primary open angle glaucoma (POAG)," stated the firm.
'Non-invasive procedure'Existing laser treatments for glaucoma - currently believed to affect 76 million people worldwide, a figure expected to grow to 112 million by 2040 - serve to reduce intraocular pressure (IOP) in the eyeball, but are regarded by ViaLase as minimally invasive, rather than non-invasive.
"The ViaLase Laser combines a femtosecond laser and micron-level, high-definition image guidance to deliver a non-invasive glaucoma treatment called femtosecond laser image-guided, high-precision trabeculotomy, or FLigHT," it explains.
"The ability to non-invasively create a conduit between Schlemm's canal and the anterior chamber is an advantage unique to the FLigHT procedure. This first-of-its-kind technology addresses an unmet need for a non-invasive procedure for patients who would benefit from a non-pharmacological, non-surgical procedure but whose therapeutic goals do not justify the risks of a surgically invasive procedure such as minimally invasive glaucoma surgery or traditional filtration surgery."
In October last year, ViaLase completed enrollment of 152 patients for a pivotal, multi-center trial of its approach. The enrolled patients will be treated with either FLigHT or selective laser trabeculoplasty (SLT), a widely used technique that works by unclogging the eye's trabecular meshwork.
ViaLase founder and CEO Tibor Juhasz, who is also a professor of ophthalmology at the nearby University of California, Irvine, said in a company release:
"We are preparing to embark on a new phase in our company's evolution - commercialization - and are incredibly fortunate to have the support, resources, financial backing, and counsel of such a high-caliber group of investors."
Previous successThe series C financing, which follows earlier rounds that landed $8 million in April 2019 and $27 million in November 2021, was led by a new investor along with strong support from current investors including Venture Investors Health Fund, Arboretum Ventures, and Falcon Vision, an ophthalmology investment platform supported by the private equity giant KKR.
Jim Adox, executive managing director at Venture Investors, commented: "ViaLase's innovative approach to glaucoma management has the potential to elevate the standard of care for glaucoma patients around the world.
"We have strongly believed in ViaLase's proven leadership, brilliant team, and pioneering technology since we wrote the first seed check to back the team."
Adox and the other investors will be hoping that Juhasz can repeat the success of his two previous ventures - IntraLase and LenSx. IntraLase, which developed a femtosecond laser approach to refractive vision correction, was acquired by Advanced Medical Optics for more than $800 million back in 2007, while LenSx and its femtosecond platform for cataract surgery was sold to Alcon Laboratories for a similar amount in 2010.
The pivotal trial now under way is aiming to back up the results of an initial study on nine patients showing no adverse events related to the FLigHT treatment and a reduction in average IOP at one and two years post-treatment.
Following that small-scale study lead investigator Thomas Samuelson from Minnesota Eye Consultants said: "The possibility of a non-incisional glaucoma treatment that safely delivers meaningful IOP reduction is an exciting one. I look forward to additional research that further validates these results, which are promising."

Comments
Post a Comment